|
Peptide structure determination is essential for understanding their biological functions and activities and also paves the way towards the rational design of peptides and analogues with pharmaceutical applications.
We recently reviewed the current knowledge about the design and NMR structural determination of α-helical and β-hairpin peptides.
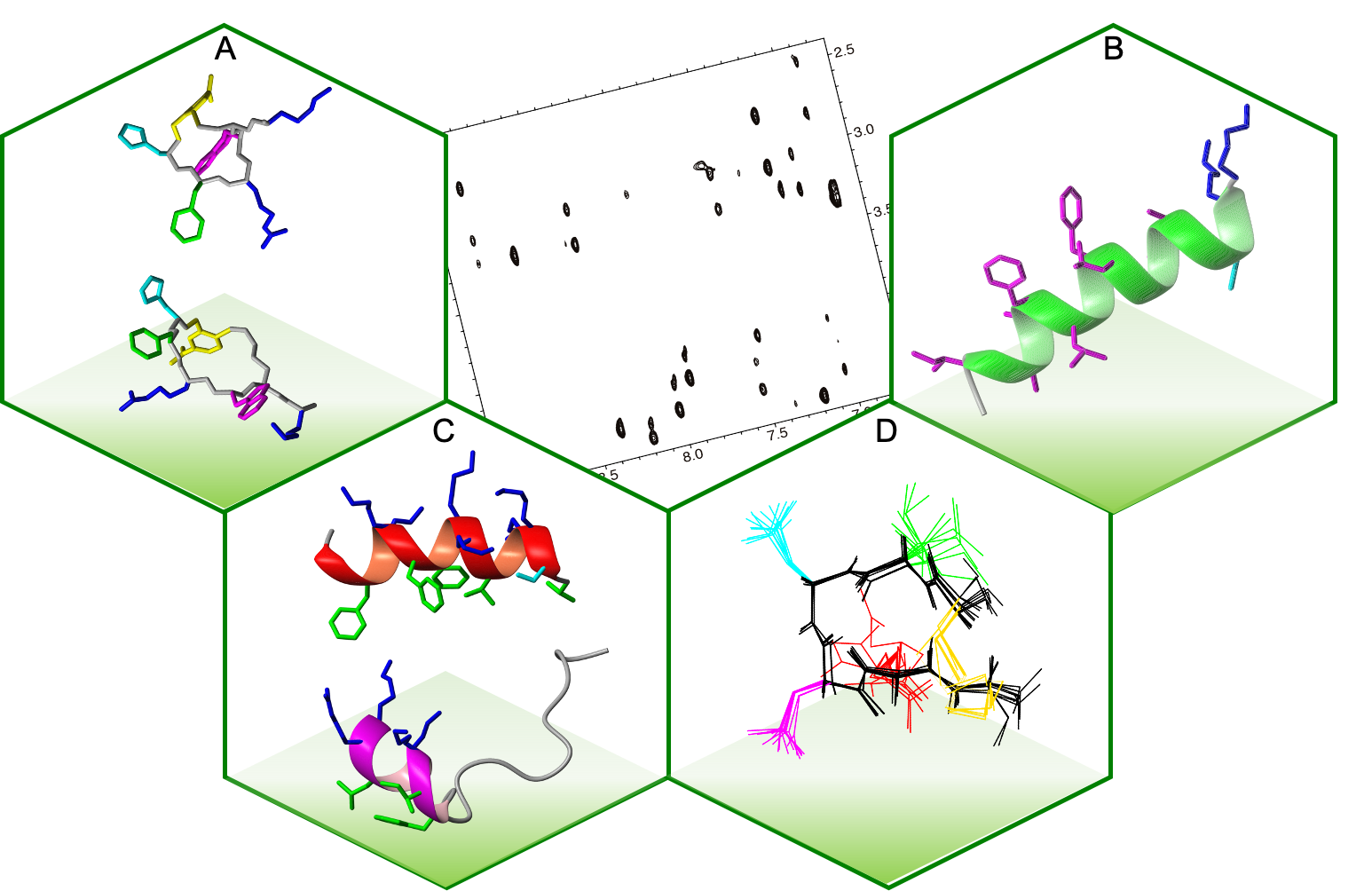
In the image, a 2D NMR spectrum is surrounded by: (A) cyclic α-MSH analogues targeting the human melanocortin receptor 1 (MC1R), which are of pharmacological interest for detecting and treating melanoma; (B) a peptide derived from the glycoprotein gp41 from the virus HIV, which is involved in membrane fusion and have immunogenic capacity against HIV; (C) two peptides derived from the antimicrobial and antitumoral crotalicidin: the N-terminal helical is inactive whereas the disordered C-terminal is active; and (D) a designed cyclic peptide able to inhibit HIV reverse transcriptase dimerization.
Publications
- D. Sandín, J. Valle, B. Chaves-Arquero, M.N. Larrosa, J.J. González, M.Á. Jiménez, E. Boix, D.Andreu, M. Torrent. Rationally modified antimicrobial peptides from the N-terminal domain of human RNase3 show exceptional serum stability (ECPs). J. Med. Chem. 64, 11472-11482 (2021). doi: 10.1021/acs.jmedchem.1c00795
- D.A. de Mendonça, M. Bakker, C. Cruz-Oliveira, V. Neves, M.A. Jiménez, S. Defaus, M. Cavaco, A.S. Veiga, I. Cadima-Couto, M. A.R.B. Castanho, D. Andreu, T. Todorovski. Penetrating the blood-brain barrier (BBB) with new peptide-porphyrin conjugates having anti-HIV activity. Bioconj. Chem. 32, 1067-1077 (2021). doi: 10.1021/acs.bioconjchem.1c00123
- P.A. Sánchez-Murcia, S. de Castro, C. García-Aparicio, M.A. Jiménez, A. Corona, E. Tramontano, N. Sluis-Cremer, L. Menéndez-Arias, S. Velázquez, F. Gago, M.-J. Camarasa. Peptides mimicking the β7/β8 loop of HIV-1 Reverse Transcriptase p51 as “Hotspot-Targeted” dimerization inhibitorsACS Med Chem Lett. 11, 811-817 (2020). doi: 10.1021/acsmedchemlett.9b00623
- P. Morales, M. A. Jiménez Design and structural characterisation of monomeric water-soluble α-helix and β-hairpin peptides: State-of-the-art. Arch. Biochem. Biophys. 661, 149-167 (2019) doi: 10.1016/j.abb.2018.11.014
- M. Ruiz-Santaquiteria, S. de Castro, M.A. Toro, H. de Lucio, K.J. Gutiérrez, P.A. Sánchez-Murcia, M.A. Jiménez, F. Gago, A. Jiménez-Ruiz, M.J. Camarasa, S. Velázquez. Trypanothione reductase inhibition and anti-leishmanial activity of all-hydrocarbon stapled α-helical peptides with improved proteolytic stabilityEur. J. Med. Chem. 149, 238-247 (2018). doi: 10.1016/j.ejmech.2018.02.071
- S. Serrano, E. Rojas, N. Huarte, D. Andreu, J.L. Nieva, M.A. Jiménez.Structure-related roles for the conservation of the HIV-1 fusion peptide sequence revealed by NMR. Biochemistry 56, 5503-5511 (2017) doi: 10.1021/acs.biochem.7b00745
- M. Morais, H. Zamora-Carreras, P.D. Raposihno, M. C. Oliveira, D. Pantoja-Uceda, J.D.G. Correia, M.A. Jiménez. NMR insights into the structure-function relationships in the binding of melanocortin analogues to the MC1R receptor Molecules 22, 1189 (2017). doi:10.3390/molecules22071189
- E. Rujas, J.M.M. Caaveiro, A. Partida-Hanon, N. Gulzar, K. Morante, B. Apellániz, M. García-Porras, M. Bruix, K. Tsumoto, J.K. Scott, M.A. Jiménez, J.L. Nieva Structural basis for broad neutralization of HIV-1 through the molecular recognition of 10E8 helical epitope at the membrane interfaceSci Rep. 6, 38177 (2016). doi: 10.1038/srep38177
- C. Borges Falcao, C. Pérez-Peinado, B.G. de la Torre, X. Mayol, H. Zamora-Carreras, M.A. Jiménez, G. Rádis-Baptista, D. Andreu Structural dissection of Crotalicidin, a rattlesnake venom Cathelicidin, retrieves a fragment with antimicrobial and antitumor activityJ. Med. Chem. 58, 8553-8563 (2015). doi: 10.1021/acs.jmedchem.5b01142
Collaborators
- David Andreu (Universitat Pompeu-Fabra, Barcelona)
- María José Camarasa (IQM-CSIC, Madrid)
- Joao D.G. Correia (Lisbon, Portugal)
- Rosario González-Muñiz (IQM-CSIC, Madrid)
- Paula Morales (IQM-CSIC, Madrid)
- José L. Nieva (UPV, Bilbao)
- Marc Torrent (UAB, Barcelona)
- Sonsoles Velázquez (IQM-CSIC, Madrid)
Financing
- PID2020-112821GB-I00 (AEI)
- B2017/BMD (CM; EU-FEDER)
|