 |
Molecular basis of the early events triggering protein diseases |
||
|
Challenge: Neurodegeneration CSIC White books:
PI: Javier Oroz
|
|||
|
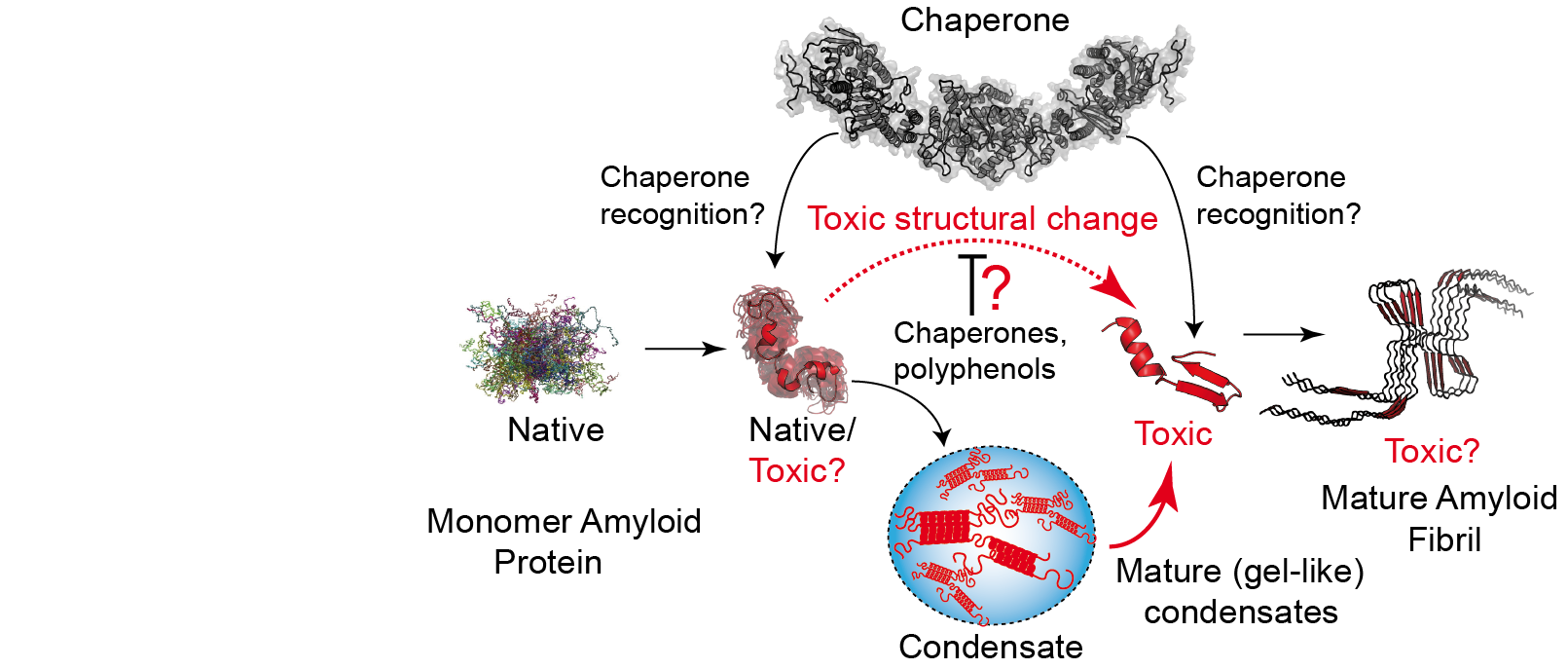
Although the critical toxic species in over 40 human diseases are misfolded proteins, we still lack a mechanistic understanding of the early structural changes in amyloid proteins that prompt their toxicity, aggregation and cell death. We study the structural basis of the gain-of-toxic action that determine protein pathologies involving TDP-43 and FUS, which are critical in the development of Amyotrophic Lateral Sclerosis. Using state-of-the art NMR and gene-editing techniques, we also describe the mechanisms used by molecular chaperones to modulate protein diseases.
In the image, structure of the pro-toxic complex formed by Hsp90-FKBP51 and Tau proteins. PublicationsOroz J, Félix SS, Cabrita EJ, Laurents DV (2020). Structural transitions in Orb2 prion-like domain relevant for functional aggregation in memory consolidation. J Biol Chem. 295(52):18122-18133. . Hervás R, Oroz J (2020). Mechanistic Insights into the Role of Molecular Chaperones in Protein Misfolding Diseases: From Molecular Recognition to Amyloid Disassembly. Int J Mol Sci. 21(23):9186. Oroz J, Chang BJ, Wysoczanski P, Lee CT, Pérez-Lara A, Chakraborty P, Hofele R, Baker JD, Blair LJ, Biernat J, Urlaub H, Mandelkow E, Dickey CA, Zweckstetter M (2018). Structure and pro-toxic mechanism of the human Hsp90/PPIase/Tau complex. Nature Communications. 9 (1): 4532. Mok SA, Condello C, Freilich R, Gillies A, Arhar T, Oroz J, Kadavath H, Julien O, Assimon VA, Rauch JN, Dunyak BM, Lee J, Tsai FTF, Wilson MR, Zweckstetter M, Dickey CA, Gestwicki JE (2018) Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat. Struct. Mol. Biol. 25 (5): 384-393. Oroz, J., Kim JH, Chang BJ, Zweckstetter M (2017) Mechanistic basis for the recognition of a misfolded protein by the molecular chaperone Hsp90. Nat. Struct. Mol. Biol. 24 (4): 407-13. Collaborators
Financing
|
|||

