 |
Polyproline II Helices in Biomolecular Condensates and amyloid formation |
||
|
Challenge: Neurodegeneration CSIC White books: Structural bases of life and evolution of macromolecular complexity (Challenge 2; CSIC white book vol.2) |
|||
|
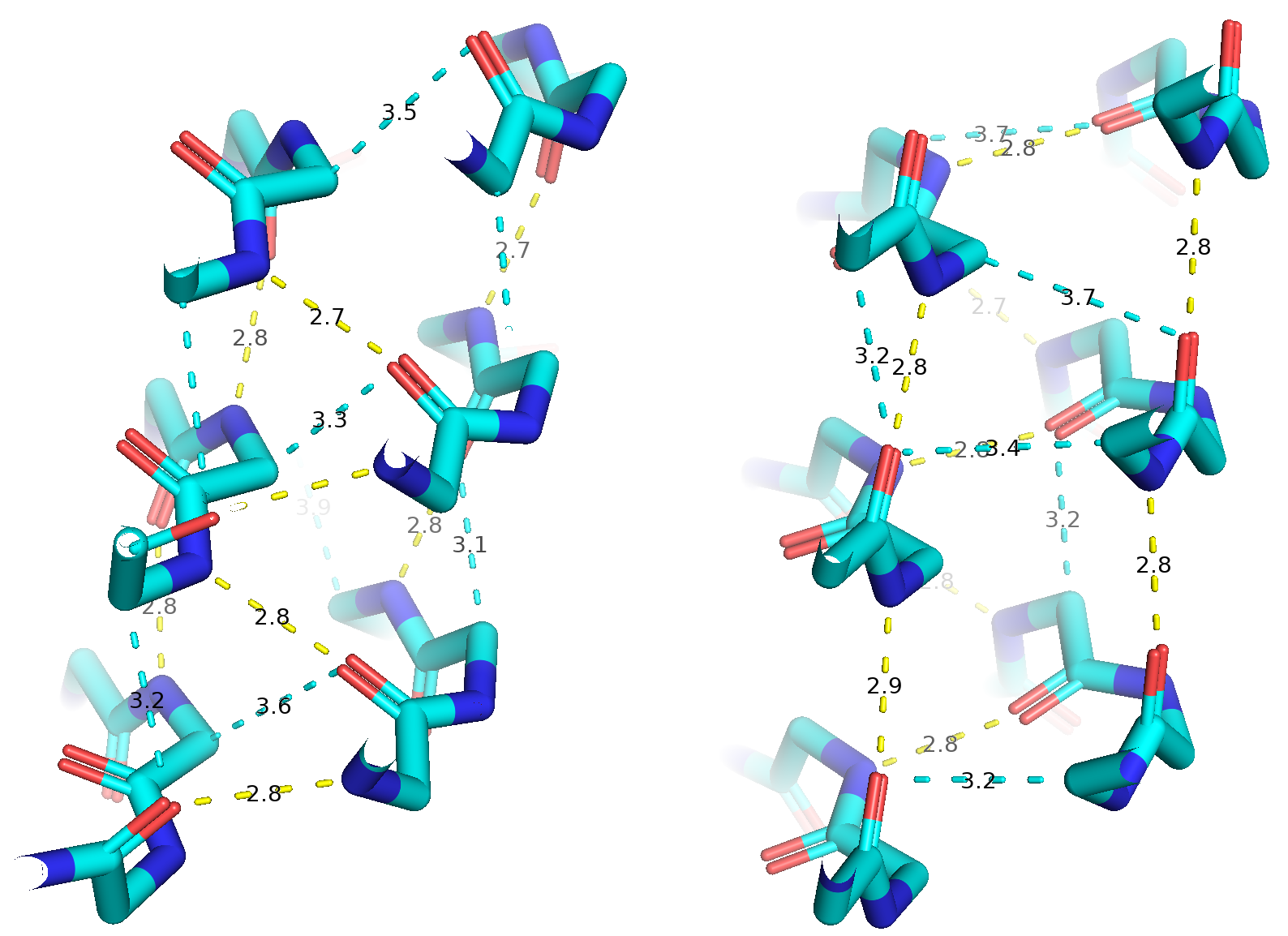
Polyproline II helices play many essential roles in biomolecular condensates like nuclear speckles and the nucleolus, but their structure and stability are not fully understood. Whereas protein design has made enormous strides in the last decade, novel structures are based exclusively on alpha helices and beta strands. In this research line, we aim to use NMR spectroscopy and computational methods to improve our comprehension of polyproline II helices’ conformational, energetics and roles in membraneless organelles, and apply this knowledge to develop novel structures and biotech tools.
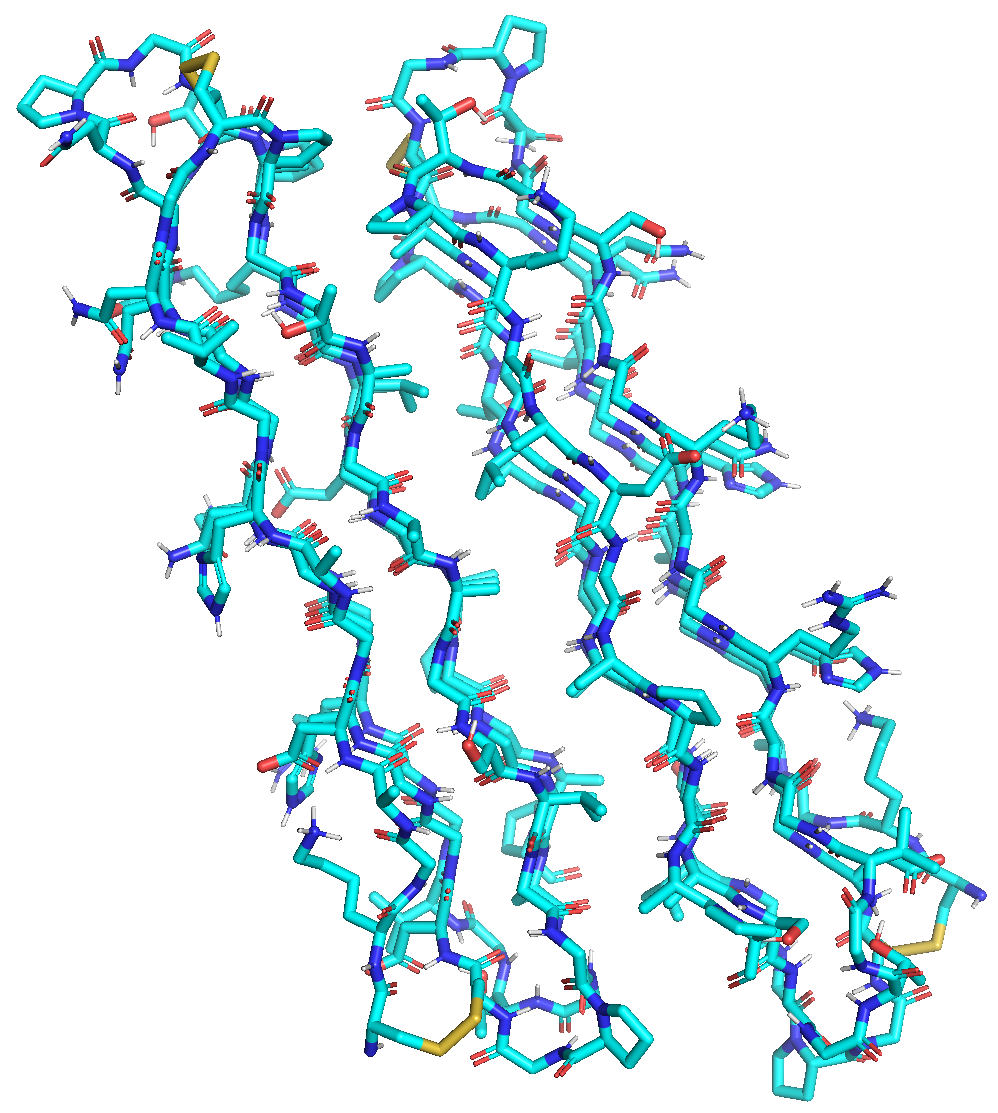
In the image, Structure of the "snow flea" antifreeze protien dimer. Publications
Collaborators Financing
|
|||